4.5 Synthesis of molecules for cell membranes of all creatures
[Chemical reaction by collision between H+ of solar wind and CO2 in the
early atmosphere]
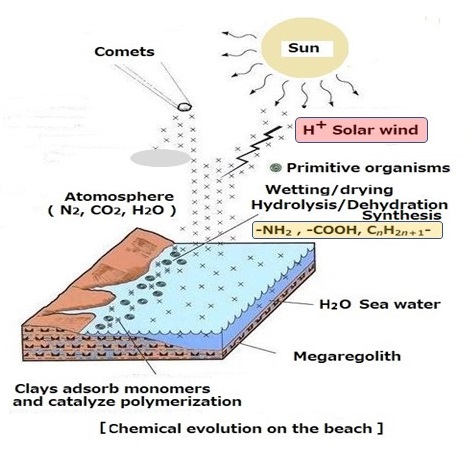
Fig.13 The cell membrane of the first creature was that was produced by
the collision of solar wind with CO2 in the atmosphere of primitive Earth.
Yuri-Miller had been carried out the experiment for origin of life by using CH4, H2 and
NH3. By the subsequent studies after Yuri-Miller’s experiment, it become
to think that the atmosphere when the first life was born was oxidizing
gases such as CO2 and NOx. But CH4, NH3 and H2 were generated by the collision
of solar wind and atmospheric molecules on the Earth.
[Hydrocarbons in liquid state floating on the sea surface of early Earth]
High-speed H+ had reacted with CO2 in the primitive atmosphere to produce water to hydrocarbons. Hydrocarbon
molecules with a small number of carbons evaporate on the Earth's surface
and stay in the sky, but hydrocarbon molecules with a higher number of
carbon atoms with a lower melting point and a higher boiling point than
the surface temperature are present on the Earth's surface as a liquid.
As shown in Table 1, hexadecan and octadecane were present in a liquid
state in the primitive global environment. The molecules spread on the
surface of the water because those are hydrophobic molecules and the specific
gravity was less than 1. Linear chained molecules of C16H34 and C16H34 were gathered on the surface of the water by hydrophobic bonding. Most
organisms' cell membranes consisted of hydrocarbons with 16 or 18 pices
of carbon atoms. Table.2 shows the data on chain of hydrocarbons (CnH2n+2) (Here, n=14~20).
Table. 2 The chain hydrocarbon (CnH2n+2) (Here, n=14~20)
| Molecule | Melting point [℃] | Boiling point [℃] | Specific gravity [20℃] | |
| Tetradecane | C14H30 | 4 to 6 | 253~257 | 0.76 |
| Hexadecane | C16H34 | 18 | 287 | 0.773~0.776 |
| Octadecane | C18H38 | 28~30 | 317 | 0.777 |
| Icosane | C20H42 | 36~38 | 343.1 | 0.7886 |
index -4.5-